Approval follows clinical trials conducted by Kansas City-based refractive surgery specialists, Durrie Vision
Staar Surgical Company recently announced the U.S. Food and Drug Administration (FDA) granted approval of the EVO/EVO+ Visian® Implantable Collamer® Lens (EVO) for the correction of myopia and myopia with astigmatism. Durrie Vision, a Kansas City-based refractive surgery center, was one of a select number of facilities in the United States to serve as aphakicclinical research site during the investigative phase of the research.
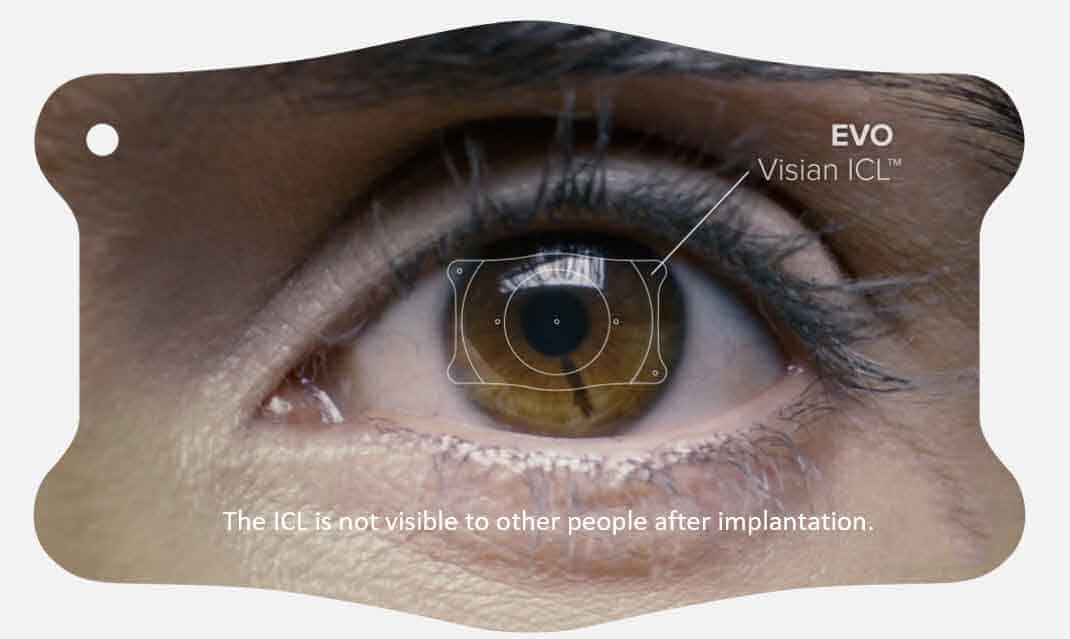
Myopia, also known as nearsightedness, affects the ability to see things clearly from a distance. An estimated 100 million U.S. adults ages 21 to 45 who have myopia are potential candidates for EVO, a biocompatible implantable lens that corrects distance vision. Implanted directly behind the iris and in front of the natural crystalline lens during a Phakic IOL procedure, the EVO ICL is the latest evolution in Visian lens technology. Phakic IOL surgery is a safe, effective alternative for prospective patients whose degree of refractive error render them ineligible for a corneal-based vision correction procedure such as LASIK.
Durrie Vision’s involvement in the research began in 2020. “As an investigator in the clinical trials, we observed first-hand the sharp visual outcomes delivered by the EVO ICL. With the FDA approval of the EVO lens, we’re thrilled to now be able to offer this exciting new technology to a broader range of patients,” said Dr. Jason Stahl, Durrie Vision CEO and refractive surgeon.
Earlier versions of the Visian ICL lens required more extensive pre-operative visits and preparation, steps that are now eliminated with the EVO ICL. This significantly improves the patient experience with regards to both comfort and time management. “After years in glasses and contacts, our patients enjoyed the ease of the Phakic procedure with the EVO ICL, along with the exceptional visual results and lifestyle benefits achieved,” said Stahl.
About Durrie Vision
Durrie Vision, located in Overland Park, KS, is a world-class refractive surgery center specializing in laser vision correction procedures to correct nearsightedness, farsightedness, astigmatism, presbyopia, and cataracts. Offering the most advanced combination of diagnostic and treatment technology available, we are dedicated to delivering exceptional results through customized vision correction procedures including SBK advanced LASIK, phakic IOL, refractive lens exchange and refractive cataract surgery. Dr. Jason E. Stahl is one of the most experienced refractive surgeons in the country with an international reputation as an innovator and leader in the field. Learn more at www.durrievision.com
About STAAR Surgical
STAAR, which has been dedicated solely to ophthalmic surgery for over 30 years, designs, develops, manufactures and markets implantable lenses for the eye with companion delivery systems. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer® Lens or “ICL,” which includes the EVO Visian ICL™ product line. More than 1,000,000 Visian® ICLs have been implanted to date and STAAR markets these lenses in over 75 countries. To learn more about the ICL go to: www.discovericl.com. Headquartered in Lake Forest, CA, the company operates manufacturing and packaging facilities in Aliso Viejo, CA, Monrovia, CA and Nidau, Switzerland. For more information, please visit the Company’s website at www.staar.com.
Important Safety Information for EVO ICL
The EVO Visian ICL lens is intended to correct/reduce nearsightedness between -3.0 D up to -20.0 D and treat astigmatism from 1.0 D to 4.0 D. If you have nearsightedness within these ranges, EVO Visian ICL surgery may improve your distance vision without eyeglasses or contact lenses. Because the EVO Visian ICL corrects for distance vision, it does not eliminate the need for reading glasses, you may require them at some point, even if you have never worn them before. Since implantation of the EVO Visian ICL is a surgical procedure, before considering EVO Visian ICL surgery you should have a complete eye examination and talk with your eye care professional about EVO Visian ICL surgery, especially the potential benefits, risks, and complications. You should discuss the time needed for healing after surgery. Complications, although rare, may include need for additional surgical procedures, inflammation, loss of cells from the back surface of the cornea, increase in eye pressure, and cataracts. You should NOT have EVO Visian ICL surgery if your doctor determines that 1) the shape of your eye is not appropriate, 2) you do not meet the minimum endothelial cell density for your age at the time of implantation, 3) you have moderate to severe glaucoma, 4) your vision is not stable; or 5) if you are pregnant or nursing.
MEDIA CONTACT:
Courtney Moilanen
Director of Marketing
Durrie Vision
8300 College Blvd., Suite 201
Overland Park, KS 66210
Phone 913-234-6301
cmoilanen@durrievision.com
################

Author Bio: Jason E. Stahl, MD
Top Doctors: https://www.castleconnolly.com/top-doctors/jason-e-stahl-ophthalmology-129cc002150
Best Cataract Surgeons: https://bestcataractsurgeons.com/cataract-surgeons/jason-e-stahl/